You are here
Decoding the structure of bone

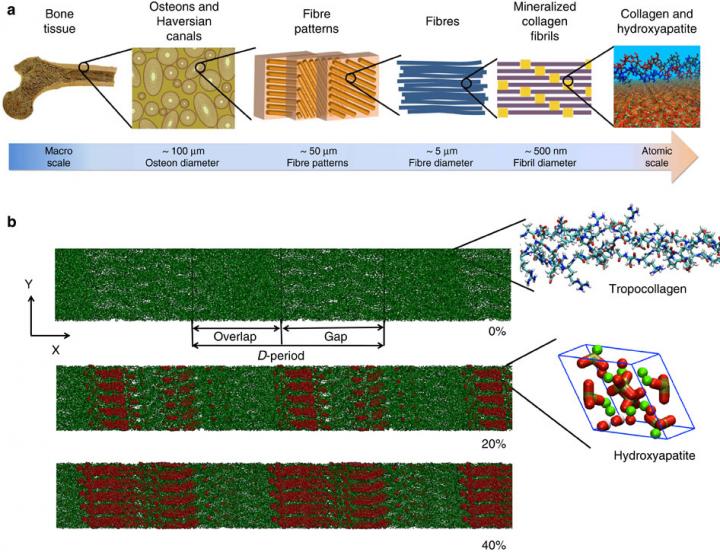
Bone is a natural composite of collagen protein and the mineral hydroxyapatite. The structure of bone is known to be important to its load-bearing characteristics, but relatively little is known about this structure or the mechanism that govern deformation at the molecular scale. Here we perform full-atomistic calculations of the three-dimensional molecular structure of a mineralized collagen protein matrix to try to better understand its mechanical characteristics under tensile loading at various mineral densities. We find that as the mineral density increases, the tensile modulus of the network increases monotonically and well beyond that of pure collagen fibrils. Our results suggest that the mineral crystals within this network bears up to four times the stress of the collagen fibrils, whereas the collagen is predominantly responsible for the material’s deformation response. These findings reveal the mechanism by which bone is able to achieve superior energy dissipation and fracture resistance characteristics beyond its individual constituents.
The findings are published in the journal Nature Communications (16 April 2013). doi:10.1038/ncomms2720
High-performance computing resources were provided by the Regione Lombardia and CILEA Consortium through the LISA Initiative, and by CINECA under the ISCRA initiative, as well as the National Science Foundation XSEDE programme (MSS090007).